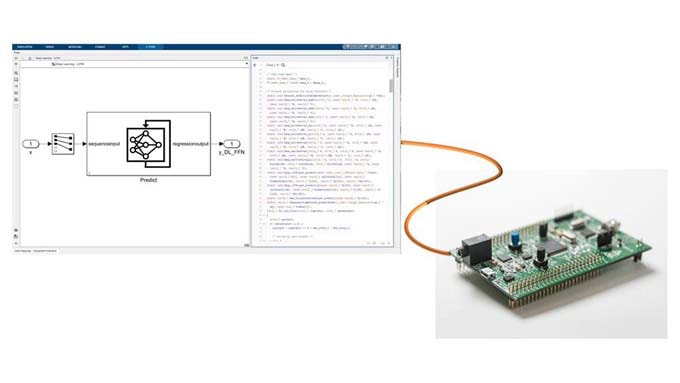
For better compliance in medical technology
Strict regulations govern quality management in medical technology. Comprehensive standards, such as DIN EN ISO 13485 or the EU's Medical Device Regulation (MDR), require seamless documentation of quality processes, which, in the event of an emergency, determine the safety and health of patients.